Serving tech enthusiasts for over 25 years.
TechSpot means tech analysis and advice you can trust.
Highly anticipated: A new device created by engineers at MIT could mark a significant advance in the management of hypoglycemia for people with type 1 diabetes. The innovation, an implantable reservoir that sits just under the skin, is designed to automatically or remotely release lifesaving glucagon when blood sugar drops to dangerous levels.
For individuals with type 1 diabetes – an autoimmune condition – maintaining stable blood sugar is a constant challenge. Rapid-acting insulin must be administered, either through injections or a pump, to help the body absorb glucose from the bloodstream after consuming carbohydrates. But judging the correct amount isn't easy, and too much can lead to dangerously low glucose levels.
Hypoglycemia, or low blood glucose, can develop quickly and, if untreated, may lead to confusion, seizures, or, in extreme cases, even death. The standard treatment in the case of an emergency is an injection of glucagon, a hormone that prompts the liver to release stored glucose into the bloodstream. Yet recognizing the onset of hypoglycemia is not always possible, particularly during sleep or for children and others who may not sense the warning signs.
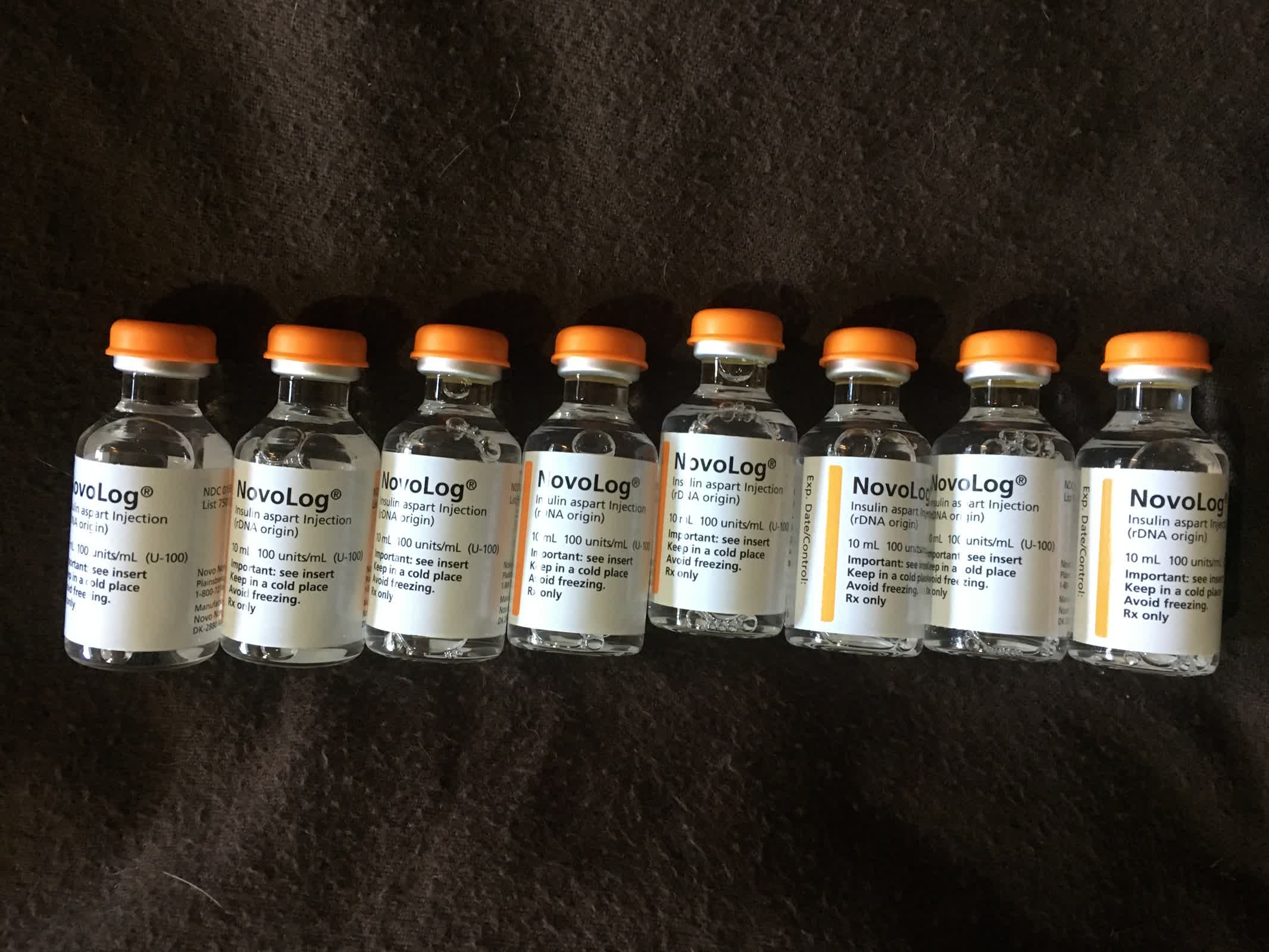
The MIT team's new device aims to bridge this gap. About the size of a quarter, the implant contains a small reservoir made from a 3D-printed polymer. This reservoir stores glucagon in powdered form, which is more stable than the liquid version and can remain viable for extended periods.
The reservoir is sealed with a shape-memory alloy made from nickel and titanium, engineered to bend and open when heated to 40 degrees Celsius.
The device can be triggered in two ways: manually by the user or automatically by a signal from a glucose monitor. It includes an antenna that responds to a specific radio frequency, activating a tiny electrical current that heats the alloy. When the temperature threshold is reached, the alloy bends, opening the reservoir and releasing the powdered glucagon, which then dissolves and enters the body.
This approach offers several advantages. Because it can interface with continuous glucose monitors, the device could release medication precisely when blood sugar falls below a safe level, even if the patient is unaware.
In laboratory tests with diabetic mice, the device was able to restore blood sugar to normal levels within ten minutes of activation. The researchers also tested the device for its ability to administer epinephrine, a medication commonly used in emergency treatment for severe allergic reactions and cardiac events. In these experiments, epinephrine was released into the bloodstream within minutes, causing a measurable increase in heart rate.
One of the challenges with implantable devices is the body's tendency to form scar tissue around foreign objects, which can interfere with their function. The MIT researchers found that their device remained effective even after fibrotic tissue had developed, successfully releasing its contents on demand.
The prototype was tested for up to four weeks in animal models, but the researchers are working to extend its functional lifespan to a year or more. This would allow the device to provide emergency protection over longer periods before needing replacement.
Looking ahead, the team plans further animal studies and hopes to begin clinical trials within three years. If successful, this technology could not only improve safety for people with diabetes but also serve as a platform for delivering other emergency medications.







 English (US) ·
English (US) ·